Neuro-MS/D Advanced Therapeutic
Transcranial Magnetic Stimulator
- advanced solution for repetitive transcranial magnetic stimulation (rTMS)
- 20 Hz stimulation with 100% intensity
- number of pulses generated during one session – up to 10000
- Neuro-MS.NET software to control magnetic stimulator
- 3-minute depression treatment protocol
- application: psychiatry, neurology

Learn more about methods and equipment
Description
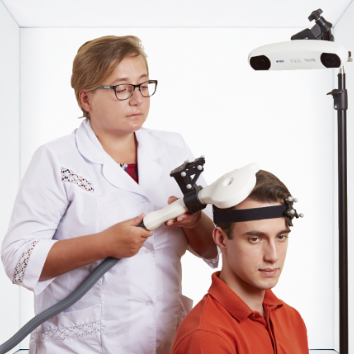
Advanced set for rTMS
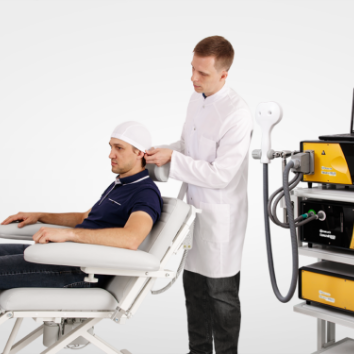
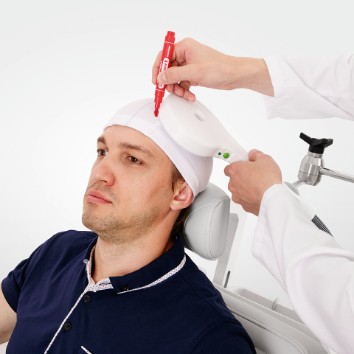
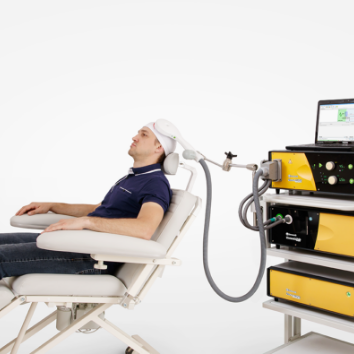
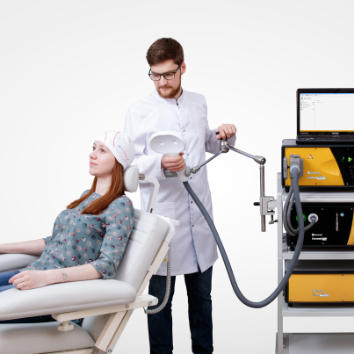
Advanced therapeutic configuration of Neuro-MS/D covers all clinical needs of rTMS practitioners. Convenient system on a trolley includes high-frequency and high-intensity stimulator with effective cooling system and convenient flexible arm for coil positioning.
20 Hz stimulation with 100% intensity
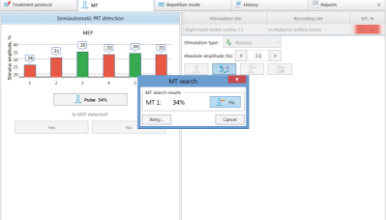
Advanced therapeutic configuration provides the possibility to increase the maximum effective stimulation frequency (the frequency when each stimulus in series has 100% intensity of motor threshold for most people) fourfold (up to 20 Hz) owing to extra power supply unit.
Research configuration provides the possibility to increase induced magnetic field by 40% and performing advanced diagnostic TMS: paired-pulse stimulation, SICI, LICI, ICF (GABAergic mechanisms) owing to expansion unit.
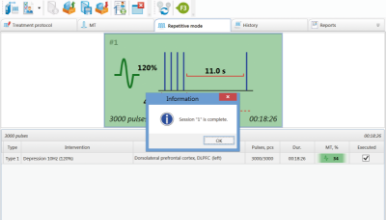
Number of pulses generated during one session – up to 10 000
The cooling unit and cooled coils (included in the delivery set) are developed to avoid overheating of coils during the long treatment session. The cooling unit itself is a tank with the cooling liquid, the pump running the cooling liquid through the coil and the fridge. The unit allows increasing continuous operation of up to 10 000 pulses without overheating. Practically, it means that stimulator can operate for hours without overheating.
Neuro-MS.NET software to control magnetic stimulator
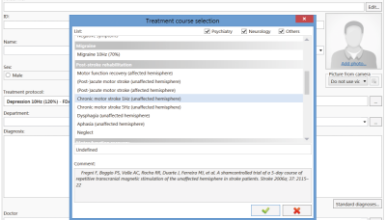
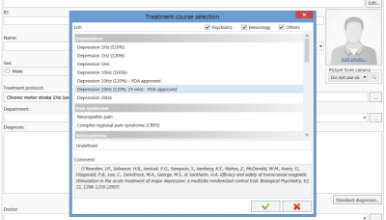
This delivery set includes the special Neuro-MS.NET software that allows customizing any stimulation protocols. This software keeps patient database, determines motor response threshold and controls the stimulation courses and sessions. You can perform the stimulation according to pre-defiend protocols and also create own ones or customize the preset stimulation programs.
In advanced therapeutic configuration the rapid stimulation protocols with up to 100 Hz frequency as well as theta-burst stimulation (TMS) are available.
Preset protocols are used for the treatment of depression, Parkinson disease, stroke, tinnitus, etc.
3-minute depression treatment protocol
Now the Neuro-MS.NET is equipped with a new short depression treatment protocol.
In accordance with the clinical tests performed by leading research hospitals in Canada*, in patients with treatment-resistant depression, iTBS was non-inferior to 10 Hz rTMS for the treatment of depression. Both treatments had low numbers of dropouts and similar side-effects, safety, and tolerability profiles. With iTBS, the number of patients treated per day with current rTMS devices can be increased several times without compromising clinical effectiveness.
So, the 3-minute treatment is as safe and effective for the depression treatment as the conventional TMS.
Application: psychiatry, neurology
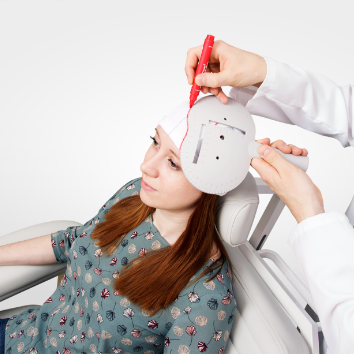
The principle of therapeutic magnetic stimulation is based on the following. The magnetic stimulator uses the magnetic pulses of short duration. The emerging high-intensity electromagnetic field easily penetrates through clothes, cranium bones and soft tissues. It exerts influence upon deep nerve centers, peripheral nerves, cerebral and spinal cord, which is inaccessible for other kinds of stimulation.
This stimulation is commonly used to treat depressions (including severe and drug-resistant), tinnitus. Besides, there are positive and encouraging results obtained at rehabilitation of post-stroke patients and other neurological and psychiatric diseases.
* Blumberger, Daniel M., et al. "Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial." The Lancet 391.10131 (2018): 1683-1692.
The support of HL7 standard allows integrating all diagnostic Neurosoft systems into the information system of a healthcare facility.Delivery Set
The delivery set can differ from country to country. Request the actual delivery set for your country from your local representative.
| Magnetic stimulator «Neuro-MS/D» (Magnetic stimulator for diagnostic and therapeutic stimulation of cerebral cortex motor zones and peripheral nervous system (with cooling and additional supply power units)) | 1 pcs. |
| License for Neuro-MS.NET software | 1 pcs. |
| Neuro-MS/D main unit | 1 pcs. |
| Patient cap (size 42-54) | 10 pcs. |
| Patient cap (size 54-66) | 10 pcs. |
| Silicone oil (canister, 3 l) | 1 pcs. |
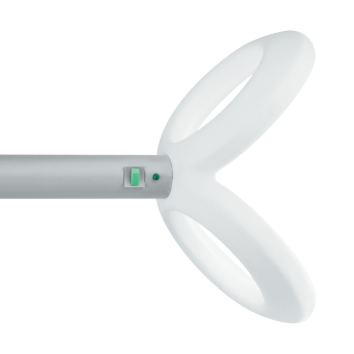
| AFEC-02-100-C cooled angulated figure-of-eight coil, 100 mm (cable 1,5 m, for Neuro-MS/D) | 1 pcs. |
| Hydraulic system adapter for coil connection to Neuro-MS/D cooling unit | 2 pcs. |
| Control cable cooling unit | 1 pcs. |
| HV cable for extra power supply unit connection to Neuro-MS/D | 1 pcs. |
| Equipotential cable | 2 pcs. |
| Monitor-computer cable (220 V, 1.8 m, 3х0.75) | 1 pcs. |
| SCZ-1 mains supply cable | 2 pcs. |
| USB cable (A-B) | 1 pcs. |
| K-3 flexible arm for coil positioning | 1 pcs. |
| K-8 coil holder (trolley/wall mounted) | 1 pcs. |
| T-4/А trolley | 1 pcs. |
| NS4D 42006 3/8" quick connect coupling | 1 pcs. |
| Technical Manual «Neuro-MS/D» | 1 pcs. |
| Warranty certificate | 1 pcs. |
| Technical Manual «Coils for Magnetic Stimulators» | 1 pcs. |
| User manual «Neuro-MS.NET (ver.2)» | 1 pcs. |
| ''Transcranial Magnetic Stimulation'' handbook by Moacyr Alexandro Rosa and Marina Odebrecht Rosa | 1 pcs. |
| Package | 2 pcs. |
| Package | 1 pcs. |
| AFEC-02-100-C coil positioning tool | 1 pcs. |
| Neurosoft equipment cover | 1 pcs. |
Options
-
Нейро-МСД_Регистрационное удостоверение_RF_23.08.2017
3,18 MB
-
Registration Сertificate_Peru
913,21 KB
-
Нейро-МСД_Регистрационное удостоверние_UZ_20.10.2020
3,05 MB
-
Neuro-MS, MS/D, MSX_Certificate of compliance_Brazil_22.10.2021
2,56 MB
-
Registration certificate_El Salvador
376,29 KB
-
Neuro-MS/D_Registration certificate_Colombia
180,59 KB
-
Выписка из реестра РЭП_Нейро-МС/Д_терапевтический расширенный
319,21 KB
-
Нейро-МС/Д_Регистрационное удостоверение_KZ_27.03.2025
673,85 KB
-
Registration Сertificate_Paraguay
306,69 KB
-
Свидетельство на товарный знак Нейро-МС/Д
793,83 KB
-
Registration certificate _Japan
506,17 KB
-
Australia certificate TMS MSD_MSX 30Mar2020
145,4 KB
-
Свидетельство о регистрации ПО Нейро-МС.NET
384,41 KB
-
Registration certificate_Neuro-MS/D_Costa Rica
243,33 KB
-
Registration certificate_Neuro-MS/D_Indonesia
3,01 MB










Neuro-MS/D Advanced Therapeutic
Neuro-MS/D Advanced Therapeutic
Neuro-MS/D Advanced Therapeutic
Neuro-MS/D Advanced Therapeutic