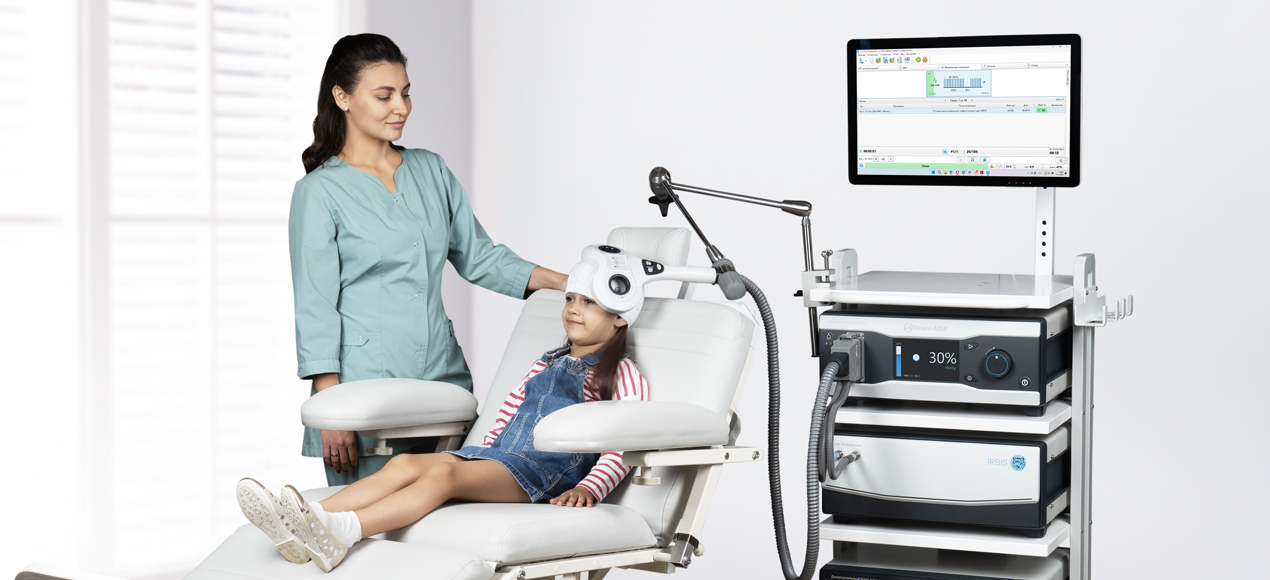
TMS for Children
Neuro-MSX
- diagnostics of nervous system disorders
- treatment of neurological and psychiatric disorders
- non-invasive and painless procedure
- pre-defined treatment protocols
Learn more about methods and equipment
Description
Transcranial magnetic stimulation (TMS) is a valuable diagnostic and therapeutic technique recommended for wide application in neurorehabilitation and functional diagnostics in adults and children.
Diagnostics of nervous system disorders
Diagnostic TMS can be used in children for direct assessment of motor pathway conduction all the way from the cortex to effector muscle. This technique allows measuring nerve conduction velocity and evaluating amplitude, latency and waveform of the obtained response. In practice, diagnostic TMS is most valuable for prognosis of functional recovery after spinal (injury, myelitis) or hemispherical (stroke, injury, other focal damage) condition.
Treatment of neurological and psychiatric disorders
TMS has proved effective for treatment of different central nervous system (CNS) disorders. This technique significantly improves the effectiveness of corrective activities targeting mental and language status.
TMS is as well effective in children with cerebral palsy (CP) undergoing neuroprotective treatment.
Application of repetitive transcranial magnetic stimulation (rTMS) in treatment of:
- autism spectrum disorder (ASD)
- perinatal CNS damage (stroke, CP)
- Tourette syndrome
- attention deficit hyperactivity disorder (ADHD)
- developmental delay (including speech delay)
- depression, OCD, addiction, anxiety
Non-invasive and painless procedure
Alternating magnetic field generated by the magnetic stimulator easily passes through clothes, skin, hair, bone and meninges. Reaching conductive structures of central and peripheral nervous systems, this field creates electric field which in its turn induces electric current that activates neurons as during electrical stimulation. However, if compared to electrical stimulation, magnetic stimulation does not cause painful sensations that usually occur due to activation of skin receptors under the electrode and does not require additional preparation time which is so important for little patients.
Pre-defined treatment protocols
Purchasing Neuro-MSX magnetic stimulator, you get the advanced software with the set of pre-defined protocols for treatment of neurological and psychiatric disorders according to the International Federation of Clinical Neurophysiology (IFCN) recommendations. Enhanced software functionality allows you to save considerable time when performing TMS.
The support of HL7 standard allows integrating all diagnostic Neurosoft systems into the information system of a healthcare facility.Delivery Set
The delivery set can differ from country to country. Request the actual delivery set for your country from your local representative.
| Neuro-MSX main unit | 1 pcs. |
| License for Neuro-MS.NET software | 1 pcs. |
| Irbis cooling unit | 1 pcs. |
| Neuro-MSX extra power supply unit | 1 pcs. |
| AFEC-03-100-C cooled angulated figure-of-eight coil, 100 mm | 1 pcs. |
| HV (high-voltage) cable for Neuro-MSX extra power supply unit connection | 1 pcs. |
| Control cable for Neuro-MSX cooling unit | 1 pcs. |
| End cap for Neuro-MSX | 1 pcs. |
| High-voltage coil connector | 1 pcs. |
| Patient cap (size 37-42) | 10 pcs. |
| Patient cap (size 42-54) | 10 pcs. |
| Patient cap (size 54-66) | 10 pcs. |
| AFEC-03-100-C coil positioning tool | 1 pcs. |
| Silicone oil (canister, 3 l) | 1 pcs. |
| Equipotential cable | 2 pcs. |
| CEE 7/7–IEC C19 mains supply cable | 2 pcs. |
| SCZ-1 mains supply cable | 1 pcs. |
| USB cable (A-B) | 1 pcs. |
| K-3 flexible arm for coil positioning | 1 pcs. |
| K-8 coil holder (trolley/wall mounted) | 1 pcs. |
| T-4/А trolley | 1 pcs. |
| Funnel | 1 pcs. |
| Key for hex drive | 1 pcs. |
| Measuring reel | 1 pcs. |
| Marker pen | 1 pcs. |
| Patient button (USB-3) | 1 pcs. |
| Technical Manual «Neuro-MSX» | 1 pcs. |
| Warranty certificate | 1 pcs. |
| Technical Manual «Coils for Magnetic Stimulators» | 1 pcs. |
| User manual «Neuro-MS.NET (ver.2)» | 1 pcs. |
| Unit package | 1 pcs. |
| Package set for Neuro-MSX main unit and cooling unit | 2 pcs. |
| ''Transcranial Magnetic Stimulation'' handbook by Moacyr Alexandro Rosa and Marina Odebrecht Rosa | 1 pcs. |
Options
-
Sokhadze EM, Lamina EV, Casanova EL, et al. (2018) Exploratory Study of rTMS Neuromodulation Effects on Electrocortical Functional Measures of Performance in an Oddball Test and Behavioral Symptoms in Autism.