Acoustic Immittance Testing
Audio-SMART
- really portable middle ear analyzer
- high-frequency tympanometry
- simple and user-friendly touchscreen interface
- all techniques in one powerful device
Learn more about methods and equipment
Description
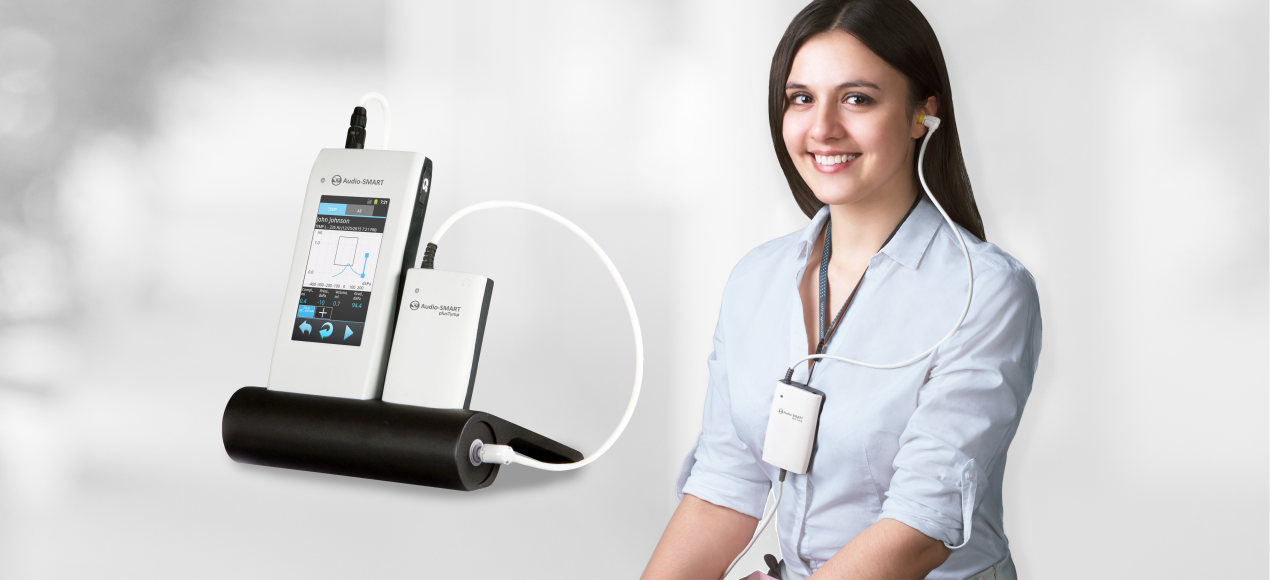
Really Portable Middle Ear Analyzer
Audio-SMART is the best solution for on-site mobile hearing testing with high diagnostic level. The system can operate in a stand-alone mode for a long time and due to its large memory nearly unlimited number of exams can be saved. Audio-SMART system is delivered in a lightweight bag, comfortable to carry and store the device and its accessories.
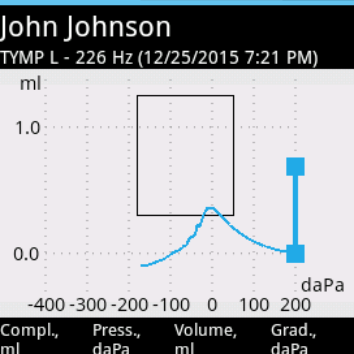
High-frequency Tympanometry
High-frequency tympanometry (1000 Hz probe tone) is recommended for hearing evaluation of newborns and infants. You can easily select the probe tone and air pressure range. During one session up to 4 tympanograms with different settings can be obtained. The system can stop examination automatically when tympanogram peak pressure is detected. It helps to reduce testing time and protect ear from overpressure.
Simple and User-friendly Touchscreen Interface
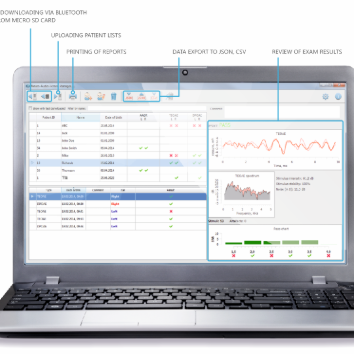
Audio-SMART has a large touchscreen display with intuitive graphical interface. Whatever function is required, it is always at hand. Enter patient’s data, start testing, review and print the results with just the touch of your finger.
All Techniques in One Powerful Device
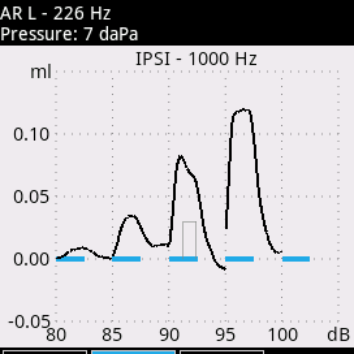
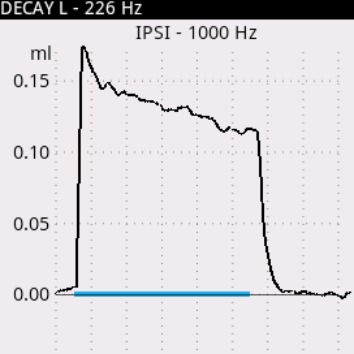
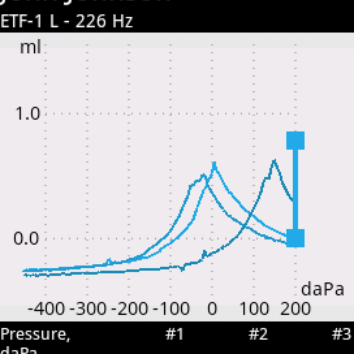
Audio-SMART is a state-of-art system that combines our experience and the best modern technologies. Developed for future needs, it meets all present-day requirements for hearing screening and diagnostics. You can perform both quick hearing screening (TEOAE, DPOAE, AABR, ABR) and complete examination of middle ear using the following tests: tympanometry, Eustachian tube function, acoustic reflex and acoustic reflex decay.
Audio-SMART is a multi-function handheld device with large color touchscreen, built-in memory card and modular Android software that can be easily customized to fit your requirements. Do not spend money on a new device, you can start with one module and then add functionality, if necessary.
The support of HL7 standard allows integrating all diagnostic Neurosoft systems into the information system of a healthcare facility.Options
-
Аудио-СМАРТ_Регистрационное удостоверение_KZ_17.08.2017
696,34 KB
-
Аудио-СМАРТ_Регистрационное удостоверение_RF_31.01.2017
5,78 MB
-
Registration certificate _Taiwan
786,21 KB
-
Выписка из единого реестра российских программ для ЭВМ ПО "Аудио-Смарт"
189,89 KB
-
Аудио-СМАРТ Австралия
999,42 KB
-
Сертификат об утверждения типа СИ в Республике Казахстан
1,54 MB
-
Выписка из единого реестра российских программ для ЭВМ ПО "Нейро-Аудио-Скрин менеджер"
189,75 KB
-
Свидетельство о регистрации ПО Нейро_Аудио-Скрин менеджер
1,91 MB
-
Свидетельство о регистрации ПО Аудио-СМАРТ
3,48 MB
-
Registration certificate _Ecuador
156,14 KB
-
Registration certificate_Audio-SMART_Indonesia
2,44 MB